Welcome to the Northumbria GP Portal!

Here at Northumbria Healthcare NHS Foundation Trust we work with GPs and a broad range of partners across Northumberland and North Tyneside to provide high quality services that meet the needs of our local communities.
To support our GPs in making referrals and to make sure that important patient information is readily available, we have created a GP Portal where you can access the latest primary care updates and keep informed about the latest events and developments.
If you have a query or if there is something that you would like to be included on this page please email emily.
Online Service to Book X-Rays
We are aware of recent issues preventing patients from accessing the online service to book an x-ray appointment via the Northumbria Healthcare patient portal.
This is now fixed. Patients can now book x-ray appointments on the patient portal here. For more information visit -www.
Please can you also update your websites with the new patient portal weblink above.
We are arranging for a replacement poster to be sent to you as the QR code in the current poster is now out of date and can’t be used. However, you can download the replacement poster via this link.
We apologise for the inconvenience caused to you and your patients.
If you require further information, please contact david.
Important information
Please see below important information that has been published in our previous bulletins.
The process for registering for entitlement and approval for non-medical referrers (NMR) is now digital which ensures it is more robust. All NMR are required to apply using the digital process to ensure compliance is up to date and held on one system.
The CQC may ask practices for your up to date plan and a list of NMR with their scope of practice.
To make things easier a model Local Plan is enclosed for reference
We have also produced the below video which explains these changes in more detail.
Please also refer to this document which explains the entitlement process for non-medical referrers (NMRs)
Please note that the deadline for non-medical referrers (NMR) to be registered is Wednesday 1 October 2025.
The Northumbria Pathology department will soon be introducing the SARSTEDT Urine Monovette collection system for all hospital and GP sites affiliated with Northumbria Healthcare NHS Foundation Trust
This collection method will replace the plain (white) and boric acid (red) universal tubes, which are currently used for Biochemistry and Microbiology urine samples.
This is a quality improvement and sustainability project, aiming to improve sample turnaround with reduced processing time in the lab, minimise use of single-use plastics, all while maintaining low costs.
The Urine Monovette System
The completely needle-free and hygienic Urine-Monovette is an all-in-one solution for urine collection and analysis that also enhances patient and user safety.
Used as a centrifuge tube, the Urine Monovette reduces turnaround time and consumable waste by preventing the use for secondary plastic containers and plastic transfer consumables in the laboratory.
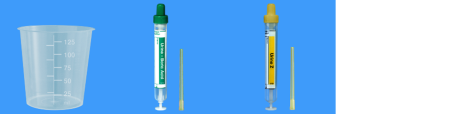
Commercially available test strips for chemical urine analysis can easily be placed into the filled Urine Monovette.
Propsal
Sarstedt are working in partnership with the Pathology Department and stakeholders at all levels across Northumbria Trust and its users to provide comprehensive training in all aspects of the Urine Monovette system. This will be achieved through direct user facing training and the use of supportive media and material; user guides and instructional videos will continue to be available prior, during and post implementation.
Urine collection: Instruction for hygenic urine collection with the Urine Monovette® - YouTube
All hospitals, community sites and GP Practices will be converting to using the Urine Monovette for biochemistry analysis and the Urine Monovette Boric Acid for microbiological analysis, with roll out commencing from the 8th September 2025.
Prior to this time, initial stocks of Monovette® products will be supplied to the user with supportive documents. Supplies will begin to be distributed week commencing the 18th August 2025.
The training of staff will commence from the 8th September 2025. Staff will be advised to use the current system until sufficient training, and supportive documents have been provided, after which they can begin using the new system.
Following the initial implementation process, ongoing training and technical support will continue to be provided.
Ongoing supplies can be ordered through the routine Pathology supplies ordering process via PathologySuppliesNSECH
Please note: the tube must be appropriately barcode labelled with an ICE label before being sent to the laboratory.
For any queries, please contact Pathology Projects at PathologyProjects
Following previous discussions with regards to GP practices requesting investigations for Sexual Health, such as Chlamydia and Gonorrhoea NAAT, and ensuring that these patients have consented and are referred to the Sexual Health Clinic for treatment and contact tracing etc, we have changed ICE to indicate consent has been received.
Where consent has been indicated TREATMENT and CONTACT tracing will be provided by the Sexual Health team where appropraite, where this is not indicated please treat the patient/contacts but we would still advice a sexual health referral.
- When requesting investigations relating to Sexual Health on ICE, GP practises will see a question asking them if the patient has consented for the Sexual Health (SH) team to contact them directly if their result is positive. GP practises will need to answer YES or NO as to whether they have discussed this with the patient and whether the patient has consented.

- If a Sexual Health test is positive, the Laboratory Information Management System (LIMS) will detect whether the answer was YES or NO when requesting in ICE and add an appropriate comment to the report stating either:
CONSENTED:
‘This patient has been recorded as giving consent to the Sexual Health Service at Northumbria Healthcare NHS Foundation Trust to contact them directly if their result is positive. The Sexual Health Service will be automatically notified to contact this patient.'
NOT consented:
‘This patient has been recorded as NOT giving consent to the Sexual Health Service at Northumbria Healthcare NHS Foundation Trust to contact them directly if their result is positive. Please consider discussing again with the patient and referring them to Sexual Health services.’
We are aware of recent issues preventing patients from accessing the online service to book an x-ray appointment via the Northumbria Healthcare patient portal.
This is now fixed. Patients can continue to book x-ray appointments on line via the NHFT Radiology Website -www.
You may have the following information / posters displayed in your practice. NHFT are arranging for a replacement poster to be sent to you as the QR code in the current poster is now out of date and can’t be used.

We apologise for the inconvenience caused to you and your patients.
If you require further information, please contact david.
Sarah Robinson, Medical Examiner has put together some top tips for completion of MCCDs, please see attached.
Please note the section below where the sole use of Frailty is not longer appropriate it can however be used where justified by listing the medical conditions contributing to the frailty.
Avoid ‘old age’ alone
Old age, ‘senility’ or ‘frailty of old age’ should only be given as the sole cause of death where all the following circumstances apply:
- the deceased was over 80 years old
- you have personally cared for the deceased over a long period - this is difficult to define, but we would suggest at least several months
- you have observed a gradual decline in your patient’s general health and functioning
- you are not aware of any identifiable disease or injury that contributed to the death
- you are certain that there is no reason that the death should be reported to the coroner
You may mention old age or frailty as a contributory cause, especially if it explains the severe effect of a condition that is not usually fatal.
You should also be aware that the representative of the deceased may not regard old age as an adequate explanation for the death and may request further investigation.
It is unlikely that patients would be admitted to an acute hospital if they had no apparent disease or injury. It follows that deaths in acute hospitals are unlikely to fulfil the conditions above. You can specify old age as a cause of death, but you should also mention in part 1 or part 2, as appropriate, any medical or surgical conditions that may have contributed to the death.
Please see the enclosed helpful guidance on death certificates - Guidance for medical practitioners completing medical certificates of cause of death in England and Wales - GOV.UK
The PMHW service is a targeted early intervention and prevention children’s mental health team in Northumberland. The primary mental health workers provide county-wide support for those children and young people aged 0-18 who are registered with a Northumberland GP.
The service provides support to those that fit the criteria for ‘getting help’ support in the Northumberland graduated approach and Thrive model.
To ensure that we are dealing with referrals in as timely and efficient manner as possible, we are asking primary care colleagues to ensure any referrals they make meet our criteria and guidelines.
To support this, a new referral form has been developed. It is now available on EMIS and SystmOne. We will not be accepting referrals made using the old form after DATE.
The form includes our updated service accessibility criteria, which can also be found here.
The updated referral form is now available in Ardens Templates for EMIS and SystmOne – please select the correct form for North Tyneside or Northumberland.
Please note the waiting time from referral is approximately 12 months and the new medical management options are not yet available in the pathway.
Please fully complete the referral details
For BMI<35 refer to Tier 2 and NHS Digital Weight Management programme NHS England » The NHS Digital Weight Management Programme
Arrhythmia present on examination:
- Clinically unstable – consider AliveCor and/or discuss with ED/Amb Care.
- Clinically Stable and present on examination:
- Significant Bradycardia (<50 bpm with no rate limiting meds)- consider AliveCor to exclude heart block
- Significant Tachycardia- consider AliveCor to exclude fast AF etc.
- If Alive Cor unavailable- Urgent 12 Lead ECG
Note - if AF confirmed with AliveCor -treat as per CKS – a 12 Lead is not needed to confirm the diagnosis but should be ordered routinely to check for structural abnormalities
Arrythmia/Palpitations- Intermittent
- Consider 24, 48 or 72 ambulatory ECG depending upon frequency of symptoms eg if daily a 24hr should capture any significant rhythm issues. If very infrequent consider AliveCor device at home via A & G to cardiology
- A Routine 12 lead ECG should also be arranged to exclude structural abnormalities or evidence of arrhythmia substrate
Chest Pain:
- Clinically unstable or acute cardiac chest pain- refer to ED
- Clinically stable non acute – Rapid Access chest pain clinic (no need for ECG pre)
- Non cardiac sounding chest pain and referral to Rapid Access chest pain clinic not planned, but ECG may provide reassurance- Request routine ECG
New Hypertension:
- Routine 12 Lead ECG indicated to exclude LVH
Heart Failure:
- Routine 12 lead ECG To be done in conjunction with BNP (providing not known to have AF) +/-ECHO
QTc Monitoring:
- AliveCor devices (6 lead) are FDA approved to allow QTc calculation
- Routine 12 lead ECG can also be used for this purpose
Ischaemic Heart Disease Risk:
- ECG has no role in predicting the risk of IHD
Cardiac Sounding Syncope :
- If cardiac syncope likely, recent, or repeated consider discussion with ED/MAC
- If bradycardia or tachycardia present follow advice above
- Consider AliveCor to identify heart rhythm disturbance
- A Routine 12 lead ECG should be arranged to exclude structural abnormalities or evidence of arrhythmia substrate
Note - AliveCor devices are approved by the NHS – there is no need to repeat purely to establish a diagnosis confirmed on Alive Cor with a 12 lead ECG. The Alive Cor ECG should be filed within the patient’s clinical record.
The RCOG have updated their guidelines on Vitamin D as below:
Current guidance for implementation endorsed by the RCOG and the NHS is that:
- All pregnant and breastfeeding women take a daily supplement containing 10 micrograms of vitamin D each day between September and March.
Pregnant and breastfeeding women with dark skin or who cover their skin a lot (for example, those of African, African Caribbean or south Asian origin) may be at particular risk of deficiency and they may consider taking a daily supplement of vitamin D, 10 micrograms all year.
Consultant stroke physician and head of service for stroke medicine, Mark Garside, would like to update you on the Brainsafe service at Northumbria. This update includes an overview of the service, information on how to refer into it, what to tell your patients to expect and who to contact if further advice is needed. Watch the short video below from Mark for more information:
Major ICE & Pathology Digital Changes – Update
We have now been LIVE with the new system for three days and wanted to firstly acknowledge and apologise for the issues that have been experienced across Primary care during the initial transition period. The changeover has been a significant piece of work with huge technical challenges and changes due to move away from a 37-year-old end of life laboratory IT system. Once embedded it is anticipated that these changes will be seen positively and please be assured we are working exceptionally hard to rectify the remaining issues as soon as possible.
1: The following are the ongoing issues we are aware of:
ICE sporadically crashing SystmOne
This issue is being actively worked through as the main priority between trust IT teams and the ICE supplier, with additional support from TPP being sought. This issue appears sporadic and localised to SystemOne connections only which makes this difficult to easily troubleshoot and identify route cause. The teams are working non-stop on this, and we will update as soon as possible.
Issues with some of the newly installed Zebra printer devices
We are aware that some surgeries have had issues with some of the Zebra printer devices and ask that all of these issues are logged with the NECS IT Service Desk who are able to remote into the machines and actively resolve these issues. These can be raised via any of the methods below and we have stressed to NECS the importance of ensuring these printers are corrected as soon as possible.
2: Additionally, we are aware of more generalised feedback and frustrations regarding the new user interface in ICE as follows:
Unnecessary additional clicks
The team are working to reduce any unnecessary clicks wherever possible and in the last couple of days have been able to reduce some of these (e.g. such as order priority which now defaults to Routine).
We are aware however that there is more work to do.
We are also actively working on reducing the burden of the below box appearing numerous times:
The cause of this box repeating is due to the move to our new laboratory system and move to sample numbering and labelling at source as opposed to the move to the new user interface, however, we are actively looking at options to stop this appearing so much.
Some Text being too small to read clearly
Our supplier has been advised of the text size issue and will look to rectify this as soon as possible in the next available release which we anticipate will be within the next month or so. In the meantime, you should be able to zoom the screen in to enlarge the text using the browser zoom options.
Test Priority
Following feedback, we have changed the test priority default to routine to prevent having to change that for all tests.
Default requesting clinician
Once logged into the system, you will see that the default requesting clinician will now be the person curently logged in to prevent the need to use the drop-down box.
3: Primary Care Results
In addition to these changes to ICE this week, we have also moved to replace the main Laboratory Information Management System (LIMS) which links to all our analytical equipment. As part of this all practices were asked to add our new laboratory provider to ensure continued result receipting from us.
We have done a huge amount of work to ensure results continue to transmit to primary care systems correctly, however, we are aware that there was an issue regarding the use of the µ symbol in some of our units of measure which SystmOne was unable to accept and as such it was not allowing these results to be filed, generating an error message.
It is important to note that this is the correct unit of measure that we were sending, however, we have now been able to translate this prior to sending to sending to Primary Care. All results from 2pm yesterday should file without issue, and we will re-transmit all results that were sent containing the above character between Monday morning and yesterday from 4pm today in order to ensure these results are captured as part of your clinical record. Unfortunately we are not able to restrict this resubmission to SystmOne surgeries only and therefore EMIS surgeries should note they will also re-receive these results. We can only apologise to EMIS surgeries that this is the case.
4: Practice Systems:
All surgeries should have completed the below prior to Monday, however, the information contained below is a reminder in the event any surgery has not yet completed this work. As a reminder, during the initial transition phase we will continue sending some results
A new provider MUST be added to each GP practice system as follows:
- SystmOne Practices: The lab will need to be selected from the national picklist (F4 directory) within SystmOne.
EDI Trader Code = 1700000723
EDI Free Part = 00011
Setup > Users & Policy > Staff & Organisation Setup
EDI Setup
Add Lab
Searching for “Northumbria Healthcare NHS FT (Pathology 2024)” should return the lab to select.
Then tick the box in the column “Allow Pathology Requesting” as per your others
Then select OK at the bottom
- EMIS Practices: Your system administrator will need to ensure the following information is added into the Workflow manager area of the system:
EDI Trader Code = 1700000723
EDI Free Part = 00011
EMIS Ball > Workflow Manager
‘Config’ in the upper ribbon > under Organisation Options > ‘Trading Partners’ > Add the new EDI Settings as a new Trading Partner
The EDI Trader and Free Part codes, as above, are merged together in EMIS as 170000072300011 which is then entered into the Trading Partner Cipher field of the ‘Messages’ section.
The following guidance has been developed by the Infection Prevention & Control Team (IPCT) at Northumbria Healthcare NHS Foundation Trust (NHCT) in order to summarise management of C.difficile cases.
RESULT MANAGEMENT
- Responsibility for management of C.difficile cases remains with the service who initiated the specimen
- For specimens sent via NHCT microbiology laboratory, the IPCT will be made aware of positive C.difficile results after Consultant Microbiologist authorisation
- Unless notified directly, NHCT IPC would not be aware of C.difficile cases not tested via NHCT laboratory
C.difficile toxin positive cases
IPC TEAM SUPPORT
- IPCT will notify GP practice in-hours (111 service at weekends/bank holidays) via telephone when toxin positive result is reported
- Patients will be sent a letter (copy to GP), information leaflet and a small card which they can carry and present to any healthcare provider in the future to notify them of their previous C.difficile status
- Approximately 24 hours after result is reported, unless otherwise notified, the team will provide IPC follow-up to the patient via telephone. This includes advice around cleanliness, hand hygiene and general principles to reduce further transmission. GP can email infectioncontrol22@nhct.nhs.uk if they do not wish IPCT to contact the patient
Glutamate dehydrogenase (GDH) positive cases
IPC TEAM SUPPORT
- IPCT will no longer notify GP practices or 111 service via telephone when GDH only positive results are reported
- The IPC team will not contact the patient unless the GP specifically requests IPC follow-up in individual circumstances. GP can email infectioncontrol22@nhct.nhs.uk if they feel the patient would benefit from IPCT follow-up
TREATMENT
- NICE guidance is available via https://www.nice.org.uk/guidance/ng199
- Following review of the above resources, if the clinician requires additional advice from a Consultant Microbiologist at NHCT this can be sought via switchboard
0344 811 8111
RELAPSE/ONGOING SYMPTOMS
- Where a patient has ongoing symptoms of infection or relapse, the case should be discussed with the Consultant Microbiologist prior to sending a repeat specimen
INFECTION PREVENTION & CONTROL REVIEW (ICR’S)
- ICR’s are completed for all C.difficile cases that are identified in both acute and community settings
- The purpose of completing ICR’s is to identify any potential areas of learning from the case
- IPCT will complete the majority of each review but may contact GP practices for additional information.
- Co-operation from all services is vital to ensure a comprehensive review is complete
- ICR’s for community cases with learning identified are shared with Integrated Care Board (ICB) colleagues
FiT testing is an effective tool in the assessment of patients with suspected colorectal cancer (CRC)
FiT < 10 (negative result) confidently excludes CRC in patients without IDA; please do not refer to Colorectal 2WW service but consider other pathways below.
If a patient meets the 2WW criteria for referral with iron deficiency anaemia (IDA) then please refer on 2WW Colorectal pathway irrespective of FiT, as FiT may be less sensitive in this context.
If a patient is FiT negative but has other concerning symptoms such as significant weight loss, then please consider 2WW referral to the Non-Site-Specific Symptoms (SNSS) service (Flow chart at end of document)
If advice or review is needed for patients who do NOT meet Colorectal or SNSS referral criteria, then please refer to the Medical Gastroenterology “Advise and Refer” (GI A&R) service rather than the Colorectal 2WW. This is found in the list of Advice and Guidance Providers on eRS.
1. Role of Fit Testing and positioning in CRC referral pathways:

FIT-Flowchart.pdf (bsg.org.uk)
*Consider SNSS Pathway (see below)
Please also see the links below (links 1-3) regarding FiT, but note the following points:
- The FiT cutoff is different for the Bowel Cancer Screening Program (BCSP) and a recent negative BCSP FiT result is not sufficient to exclude CRC in symptomatic patients.
- FiT CAN be used in the context of rectal bleeding – please see link 2 below.
- Patients can be referred under suspected cancer pathways if they are unwilling or unable to perform FiT testing at home. Please indicate if there might be any implications for assessment e.g. unwillingness to undergo rectal examination, severe frailty
2. What to do if FiT is negative and SNSS pathway is not triggered?
A FiT result < means that CRC confidently excluded and referral under the Colorectal Suspected Cancer pathway is not indicated. Please consider other cancer pathways (esp pancreatic) if there are other red-flags such as weight loss (SNSS pathway)
Consider other causes and first line investigations to assess, noting that in a significant percentage of patients the diagnosis and treatment does not require a patient undergo colonoscopy.
Possible Cause
Clues and Investigations
Likely Next Steps
Functional bowel problems / Dietary effects
Negative investigations
Diagnostic criteria
Management as per NICE guidance (Link 4)
Consider dietary management (Link 5 or dietetics referral)
FODMAP diet only under supervision of dietician
Drug side effect
Consider common culprits:
NSAID
PPI
Metformin
Antibiotics
SSRI
Most medications have GI side effects
Adjust medications where possible.
Try Metformin MR preparation
Microscopic Colitis
Profuse watery diarrhoea day and night
Often in middle aged females
May have history of medications as per drug side effects, but more extreme symptoms
If suspected:
Stop culprit medications
Trial of loperamide
Refer to GI A&R for consideration of colonoscopy and biopsy
Bile Acid diarrhoea / Post cholecystectomy diarrhoea
Watery diarrhoea which may mimic IBS
Often no association with eating
Onset of symptoms post cholecystectomy.
Please see Link 6
Refer GI A&R if suspected.
Treatment may include bile acid sequestrant (e.g., cholestyramine) often with confirmatory medical physics scan (SeHCAT – Medical Physics scan at Freeman Hospital) for confirmation
Inflammatory Bowel Disease
Suggestive symptoms and investigations:
Weight loss
Inflammatory symptoms
High crp
Extra-intestinal manifestations
The key investigation is a raised Faecal Calprotectin. IBD is incredibly unlikely if this is negative.
If FCP is positive, then refer to GI A&R for likely direct-to-test colonoscopy.
Consider other causes of raised FCP however – NSAID use, GI infection prior to referral.
Coeliac Disease
Positive TTG / EMA
Referral for OGD with D2 biopsies
“Other”
Multiple other unusual conditions which may be considered e.g. pancreatic exocrine insufficiency.
Consider tests such as faecal elastase, but only if reasons to suspect chronic pancreatitis. Not recommended as a screening test in all cases of diarrhoea.
Discuss with GI A&R
1. Use Of GI A&R
We are very happy to discuss any cases you are unsure about, even if a clinic appointment or scope is not required.
Please see Link 7 for suggestions on pre-referral investigations.
Please include as much clinical detail in your referral as possible, including a copy of the 2WW referral if that was the original pathway used.
Specific information that can be helpful might include:
- Previous investigations
- Medication history
- Patient concerns, and willingness to come “direct to test”
- Any information to help us identify the most suitable clinic – location, suitability for telephone or video consultation, morning of afternoon appts etc.
We hope this brief summary, which is not intended to replicate or replace any existing clinical documents provides a few pointers.
If you have any specific queries about the Colorectal Pathway then please email Iain.McCallum@nhct.nhs.uk
If you have any comments or suggestions for the GI A&R pathway then please contact matthew.
Links:
1: GI and Colorectal - Northern Cancer Alliance Northern Cancer Alliance
General Advice regarding GI cancer referrals
2: https://
Specific advice and information regarding the role of FiT testing
Letter describing the role of FiT testing and the reassurance value of a negative test.
5: Overview | Irritable bowel syndrome in adults: diagnosis and management | Guidance | NICE
7: Diarrhoea - adult's assessment | Health topics A to Z | CKS | NICE
Non-site specific symptoms lead specialist nurse, Rachel Beaufort Jones would like to update you about the non-site specific symptoms service which went live in December 2022 to referrals in Northumbria. Please see the video for more information from Rachel.
Prescribing in pregnancy – most of these (low dose folic acid, aspirin, iron) should be available over the counter but occasional a prescription may be needed. Common scenarios are considered below – Sept 2023
For general advice on prescribing in pregnancy/patient information, refer to the ‘bumps’ website by UKTIS: bumps - best use of medicine in pregnancy (medicinesinpregnancy.org)
Iron supplementation
- Only recommended to give OD where anaemia is confirmed or at risk of iron deficiency anaemia (see table 1 in link below) ie 200mg Ferrous Sulphate or 210mg Ferrous Fumerate a day
· https://
Aspirin -150mg at night
- Community midwives will do risk assessment. Need to start at 12-16 weeks and continue until 36 weeks
- Hypertension in pregnancy: diagnosis and management (nice.org.uk)
- Summary of indications from this guidance is below:
Advise pregnant women at high risk of pre-eclampsia to take 150 mg of aspirin at night from 12 weeks until the birth of the baby. Women at high risk are those with any of the following:
- Hypertensive disease during a previous pregnancy
- Chronic kidney disease
- Autoimmune disease such as systemic lupus erythematosus or antiphospholipid syndrome
- Type 1 or type 2 diabetes
· Chronic hypertension
- Also recommended if previous fetal growth restriction
Advise pregnant women with more than 1 moderate risk factor for pre-eclampsia to take 150 mg of aspirin at night from 12 weeks until the birth of the baby. Factors indicating moderate risk are:
- Nulliparity
- Age 40 years or older
· Pregnancy interval of more than 10 years
- Body mass index (BMI) of 35 kg/m2 or more at first visit
· Family history of pre-eclampsia
- Multiple pregnancy
Folic Acid and Vitamin D- For all pregnant women
- Vitamin D 10 micrograms/day (on its own/ part of a pregnancy multi-vitamin)
- Low dose folic acid 400 micrograms (from 3 months prior to conception to 12 weeks)
High dose Folic Acid- 5mg OD
Needs to be prescribed (from 12 weeks pre-conception and until 12 weeks gestation) IF:
- History of either patent with a neural tube defect
- Family history of neural tube defects
- Previous pregnancy affected by a neural tube defect
- Type 1 or type 2 diabetes
- Epileptic
· Taking anti-retroviral medicine for HIV
- BMI >30
Low Molecular Weight Heparin
Assessed by community midwife using criteria below in any women and weight dependent. Local agreement is for hospital supply of LMWH:
- With four or more current risk factors shown in Appendix I and Table 1 (other than previous VTE or thrombophilia) should be considered for prophylactic low-molecularweight heparin (LMWH) throughout the antenatal period and will usually require prophylactic LMWH for 6 weeks postnatally, but a postnatal risk reassessment should be made.
- With three current risk factors shown in Appendix I and Table 1 (other than previous VTE or thrombophilia) should be considered for prophylactic LMWH from 28 weeks and will usually require prophylactic LMWH for 6 weeks postnatally but a postnatal risk reassessment should be made.
- With two current risk factors shown in Appendix I and Table 1 (other than previous VTE or thrombophilia) should be considered for prophylactic LMWH for at least 10 days postpartum.
- Admitted to hospital when pregnant (including to the gynaecology ward with hyperemesis gravidarum or ovarian hyperstimulation syndrome) should usually be offered thromboprophylaxis with LMWH unless there is a specific contraindication such as risk of labour or active bleeding.
3 New Gynaecology pathways are available following feedback from GP's about the most appropriate place to refer patients with the following conditions:
- Pre-Menopausal Abnormal Uterine Bleeding
- Benign Ovarian Cysts
- Pelvic Pain
Please use the associated template referrals These are available via Ardens or your clinical system in CDRC forms or searching by title in letters template.

You may have noticed that recently that the respiratory reports which have isolated Haemophilus have a new comment for the dosage of amoxicillin and coamoxiclav.
“If amoxicillin oral preparation is being considered, use amoxicillin 1 gm PO TDS. Please adjust the dose if clinically indicated, (e.g., renal impairment), and for children, use the maximum recommended dose in cBNF for the indication.”
“If coamoxiclav oral preparation is being considered, please use coamoxiclav 625 mg TDS Po with amoxicillin 500 mg TDS PO. Please adjust the dose if clinically indicated, (e.g., renal impairment), and for children, use the maximum recommended dose in cBNF for the indication.”
This has raised concerns as it may increase or even double antibiotic use. While I agree with that concern, the rationale for this is explained below.
Background:
Northumbria microbiology laboratory is moving from the US standard of sensitivity test (CLSI) to the European Standard (EUCAST).
Working with the EUCAST standard will align us with most UK laboratories as almost all of them have changed to EUCAST.
What is recommended in the EUCAST:
According to EUCAST standard, we are expected to report the sensitivities as sensitive (S), resistant (R) and SIE (Sensitive, increased exposure).
This SIE is similar to the previous intermediate sensitivity (I). Our previous interpretation of the intermediate (I) category was –“Treatment using an antibiotic with intermediate sensitivity may be adequate in this situation, but please assess clinical response. If no symptom improvement, suggest using another agent to which the organism is sensitive.”
However, EUCAST standards suggest that if we find an organism to be SIE, we may only use this antibiotic where it is expected to achieve high concentration, and we must use a higher dose.
What changes have we made in the laboratory?
To help with the decision-making process at the clinical level, we have made these changes
- we have changed all the SIE to sensitive (S) to reduce confusion – sensitivity test report will only have two categories – sensitive (S) and resistant (R).
- If we find any antibiotic in the SIE category, we report these with an interpretative comment suggesting the appropriate dose (the recommended higher dose by EUCAST).
- Microbiologists will assess whether a particular antibiotic can reach a higher concentration in the infection site and only release it if it can.
What will you find on the report?
The report will only have sensitive or resistant categories. No intermediate.With some antibiotics, you may find a comment recommending a higher dose of antibiotics.
Please note - this is advice only. The decision of antibiotic choice and dose should always be taken in the clinical context.
What if a patient is already on a standard dose amoxicillin or coamoxiclav and responding to the treatment?
All the advice and interpretive comments should be taken in the clinical context. If a patient is improving on a standard dose regimen, it can be continued, and patient can be observed clinically.
Will there be any more changes like this?
Yes. We are in the process of moving to the EUCAST standard and because of that certain antibiotics will have recommended higher dose. We will use interpretative comments to advise you on the dose.
Dr Suryabrata BanerjeeConsultant and clinical lead, Microbiology
Northumbria Healthcare NHS Foundation Trust
All GPs in Northumberland and N Tyneside should have access to CT head and MRI brain where needed- CT and MRI should be requested using the following criteria:
Head MRI for:
- Investigation of progressive, sub‑acute loss of central neurological function- Urgent
- Investigation where suggested by Neurology for MS
- Investigation of First Focal Fit
Provided discussed directly with a GP in the case of other health professionals working in primary care
Head CT for:
- Investigation of headaches where a malignancy needs excluding- Urgent
- Investigation of Dementia or Personality Change
The way Paediatric Physiotherapy referrals within Northumbria Healthcare are processed is changing.
A new Paediatric Physiotherapy dropdown box on ICE for all health professionals will be available to refer straight into. These changes will be happening from the beginning of August.
If you have any questions regarding this change, please contact: mel.
Dyspepsia is a common symptom usually caused by Functional Dyspepsia (FD) rather than any structural UGI pathology. It is often difficult to know how to treat and investigate patients who do not meet 2WW criteria but who do not respond to H Pylori eradication and PPI.
This document is to support management of patients who do not fulfil 2WW UGI criteria (including for suspected pancreatic cancer) and does not change management in these cases.
Key Messages
- In most cases investigations for dyspepsia such as UGI endoscopy will be normal.
- The commonest cause of dyspepsia in non-2WW referrals is Functional Dyspepsia (FD).
- FD may be underpinned by complex lifestyle and psychosocial factors including weight, diet and mental health.
- UGI Endoscopy may be appropriate in those > 55 yrs with resistant dyspepsia, or those > 40 yrs with particular risk factors for UGI malignancy but is otherwise of limited value.
- If H Pylori eradication and PPI / H2RA are ineffective then a trial of low dose amitriptyline should be considered.
- Please see references below for further information
Recommendations for Primary Care
- We are keen and happy to discuss any non-2WW patients with dyspepsia who are proving difficult to treat or are causing concern.
- Please direct any such referrals for dyspepsia, in patients who do not meet 2WW criteria, to GI Advise and Refer (A&R) rather than UGI Surgeons (found as an A&G Provider in eRS)
- British Society of Gastroenterology guidelines recommend that…
“… the diagnosis of FD, its underlying pathophysiology and the natural history of the condition, including common symptom triggers, should be explained to the patient. FD should be introduced as a disorder of gut–brain interaction (DGBI), together with a simple account of the gut–brain axis and how this is impacted by diet, stress, cognitive, behavioural and emotional responses to symptoms and post-infective changes”.
- Please reinforce the benefits of lifestyle change including weight loss, sensible eating and regular exercise - these are vital components in management of FD
- Please be aware that an UGI endoscopy will often not be useful or recommended except in the groups described in Key Messages above; and that treatment advice only may be suggested.
- Consider high dose PPI (e.g. esomprazole 40mg BD) if standard doses are ineffective and check that the am dose is being taken 30 minutes before first meal of the day
- A trial of amitriptyline for FD may be recommended if first line treatment has not been helpful and it may be reasonable to offer this prior to GI referral.
- Please see links below for further information
Any Questions?
If you have any queries about this, please contact:
Matthew.
Iain.
References:
- BSG Guidance on the management of dyspepsia
https://
- NICE Suspected cancer pathways
https://
- Patient UK information for sufferers of FD
https://
- How to take omeprazole (links to other PPI also available)
https://www.nhs.uk/medicines/omeprazole/how-and-when-to-take-omeprazole/
The Vasectomy Service at the Northumbria Sexual Health Service operates an open referral system. Gentlemen can refer themselves by ringing
The Vasectomy Service at the Northumbria Sexual Health Service operates an open referral system. Gentlemen can refer themselves by ringing
On contacting our service, an initial counselling appointment will be offered and consent secured prior to the operation date. Any necessary follow up care and post-operative fertility testing will be included in the service delivery. Operations are now conducted in the purpose-build minor surgery unit in Morpeth NHS Centre.
GP Excellence Through Collaboration

Our GP Excellence Through Collaboration event is an educational conference and a chance to give service updates to GPs across Northumberland and North Tyneside. Those who attend the event will receive CPD points and have the opportunity to build relationships with trust consultants, as well as network with their peers.
Our next GP Excellence Through Collaboration event will be held on Wednesday 19 November at the Northumbria Health and Care Academy. Please save this date in your diary and we will be in touch soon regarding registration information.
Excellence Through Collaboration
GP Bulletins
We find that the best way to keep in touch with GPs across our patch is through a regular bulletin. We send these bulletins out on a monthly basis and if you have any information you would like to be included in future bulletins or you would like to sign up to the mailing list for this bulletin please email emily.
Please see below for past bulletins:
Useful links
You can also see further information on our referral processes here.
GP hotline information can be found here.
Latest OPD times by speciality can be found here
Haematology Clinical Guidelines relevant to primary care
Please see below Haematology Clinical Guidelines relevant to primary care.
POLYCYTHAEMIA IN ADULTS – January 2022
Myeloma and monitoring of MGUS v3
